 [an error occurred while processing this directive]
[an error occurred while processing this directive]
 [an error occurred while processing this directive]
[an error occurred while processing this directive]

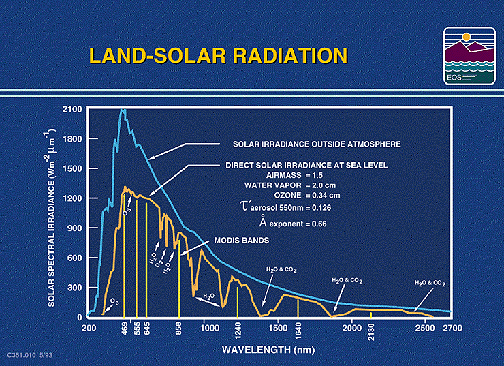
The amount of solar radiation reflected from land and sea surfaces,
as well as the amount absorbed, depends partly on that portion
of the spectral distribution of transmitted irradiant energy from
the Sun that finally reaches these surfaces. In the Introduction,
it was stated that this radiance rises rapidly to a peak at 0.48
µm, then trails off to near zero through wavelengths out to ~4.0
µm. That is confirmed in the plot shown below that also shows
many of the principal water, carbon dioxide, and oxygen absorption
bands.
A thermal sensor picks up radiant emitted energy from a surface
target heated through radiation (solar insolation and sky radiance),
convection (atmospheric circulation) and conduction (through the
ground). Thus, most sensed heat from surfaces has its origin in solar illumination,
that varies with both diurnal and seasonal changes as well as
cloud cover, but there is also a small, nearly constant contribution
from internal heat flux from the Earth's interior (much of this
is due to thermal inputs from radioactive decay). Heat is transferred
into and out of near surface layers owing to external heating
by the thermal processes of conduction, convection, and radiation.
Heat Capacity; Thermal Conductivity; Thermal Inertia
A primary objective of temperature measurements and related thermal responses is to infer something
about the nature of the composition and other physical attributes
of materials at the Earth's surface (and, in its atmosphere).
For any given material, certain characteristic internal properties
play important roles in governing the temperature of a body at
equilibrium with its surroundings.
These properties include:
- Heat Capacity (C): The measure of the increase in thermal energy content (Q)
per degree of temperature rise. It is given in cgs units of calories
per cubic cm. per degree Centigrade, and its denotes the capacity
of a material to store heat (recall from physics that a calorie
[cal] is the quantity of heat needed to raise one gram of water
by one degree Centigrade). Heat capacity is calculated as the
ratio of the amount of heat energy, in calories, required to raise
a given volume of a material by one degree Centigrade (at a standard
temperature of 15° Centigrade.) to the amount needed to raise
the same volume of water by one degree Centigrade. A related quantity,
specific heat (c), is defined as
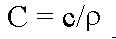 (units: calories per gram per degree Centigrade) where
(units: calories per gram per degree Centigrade) where 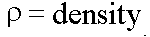 ; this associates Heat Capacity to the thermal energy required
to raise a mass of 1 g(ram) of water by 1 degree Centigrade.
; this associates Heat Capacity to the thermal energy required
to raise a mass of 1 g(ram) of water by 1 degree Centigrade.
- Thermal Conductivity (K): The rate at which heat will pass through a specific thickness
of a substance, measured as the calories delivered in 1 second
across a 1 centimeter square area through a thickness of 1 cm
at a temperature gradient of 1 degree Centigrade (units: calories
per centimeter per second per degree Centigrade)
- Thermal Inertia (P): The resistance of a material to temperature change, indicated
by the time dependent variations in temperature during a full
heating/cooling cycle (a 24-hour day for the Earth); defined as
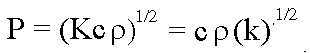 (k is a term, related to conductivity K, known as thermal diffusivity, in units of calories per centimeter squared per square root
of degree Centigrade seconds ). P is a measure of the heat transfer
rate across a boundary between two materials. e.g., air/soil.
Because materials with high P possess a strong inertial resistance
to temperature fluctuations at a surface boundary, they show less
temperature variation per heating/cooling cycle than those with
lower thermal inertia.
(k is a term, related to conductivity K, known as thermal diffusivity, in units of calories per centimeter squared per square root
of degree Centigrade seconds ). P is a measure of the heat transfer
rate across a boundary between two materials. e.g., air/soil.
Because materials with high P possess a strong inertial resistance
to temperature fluctuations at a surface boundary, they show less
temperature variation per heating/cooling cycle than those with
lower thermal inertia.
Some characteristic values of these intrinsic thermal properties:
|
Water |
Sandy Soil |
Basalt |
Stainless Steel |
| K |
0.0014 |
0.0014 |
0.0050 |
0.030 |
| c |
1.0 |
0.24 |
0.20 |
0.12 |
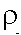 |
1.0 |
1.82 |
2.80 |
7.83 |
| P |
0.038 |
0.024 |
0.053 |
0.168 |
The interpretation of thermal data and images depicting temperature
distribution over an area is not a simple matter. In many instances,
efforts must be confined to looking for patterns of relative temperature
differences rather than the absolute values because of the many
complex factors that make quantitative determinations difficult,
such as:
- Number and distribution of different material classes in the instantaneous
field of view
- Variations in the angle of thermal insolation relative to sensor
position
- Dependency of thermal response on composition, density and texture
of the materials
- Emissivities of the surface materials
- Contributions from geothermal (internal) heat flux; usually small
and local
- Topographic irregularities including elevation, slope angle, and
aspect (surface direction relative to Sun's position)
- Rainfall history, soil-moisture content, and evaporative cooling
effects near surface
- Vegetation canopy characteristics, including height, leaf geometry,
plant shape
- Leaf temperatures as a function of evapotranspiration and plant
stress
- Near surface (1 to 3 meters) air temperature; relative humidity;
wind effects
- Temperature history of atmosphere above surface zone
- Cloud-cover history (during heating/cooling cycle)
- Absorption and re-emission of thermal radiation by aerosols, water
vapor, air gases
Some factors have fixed or constant effects; others vary with
each sensor overpass. It may be possible to correct for the influence
of some of the variable factors but this is difficult to do routinely.
Measurements made at isolated individual points in a scene and
extrapolated to the general scene have limited validity.

[an error occurred while processing this directive]

[an error occurred while processing this directive]

Code 935, Goddard Space Flight Center, NASA
Written by: Nicholas M. Short, Sr. email: nmshort@epix.net
and
Jon Robinson email: Jon.W.Robinson.1@gsfc.nasa.gov
Webmaster: Bill Dickinson Jr. email: rstwebmaster@gsti.com
Web Production: Christiane Robinson, Terri Ho and Nannette Fekete
Updated: 1999.03.15.

