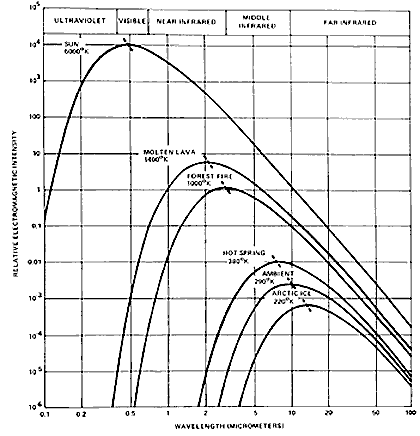

The above figure (shown earlier on page 8-8) plots spectral wavelength vs. emitted radiance (as intensity) from thermal radiators at various peak radiant temperatures ranging from that of the Sun through the Earth's surface (average ambient temperature) to sea ice . The hotter the radiating body, the greater is the radiance (intensity on the ordinate) over its range of wavelengths and the wavelength at which peak emission occurs moves to shorter wavelengths. The relation between peak wavelength and radiant body temperature is known as the Wien Displacement Law:
where is the wavelength at maximum radiant emittance and T is the absolute temperature
in degrees Kelvin ( °C + 273). (The constant, 2898 is in units of µm °K; it is also given as [rounded off] 0.29 cm °K.) For the Sun, with a photospheric
radiant temperature of ~6000 °K, this peak is in the visible
(ca 0.58 µm); a forest fire peaks around 5.0 µm; the Earth as
observed from space peaks within the 8-14 µm interval.
The radiant flux Fr emanating from a body is related its internal (kinetic) temperature Tk by the Stefan-Boltzmann Law, which in simplified form is given as Fr = sTk4, where s (often given by the Greek letter, small sigma) is a constant given as 5.67 x 10-12 W(atts) × cm-2 × ¡K-4. Strictly, this equation holds only for perfect blackbodies.
The quantity of radiant emission, and thus the effective temperature that is sensed, is also controlled by the emissivity
of the object in the spectral region of interest. Emissivity
is a dimensionless number that expresses the ratio of the radiant
flux of a real material FR to the radiant flux of a perfect blackbody FB (one that completely absorbs incoming radiant energy, with none being partitioned into transmittant or reflectant components), or FR/FB =
. It
is a measure of the efficiency of emitted radiance of any real
body to that of a perfect radiator (for which
= 1.000). Values of
vary from 0 to 1 and are spectrally dependent, i.e., can change
with
![]() . Here is an example comparing the spectral radiant emittance of the common mineral quartz to a perfect blackbody when both are at thermal equilibrium
at a given temperature (here, at 600 °K).
. Here is an example comparing the spectral radiant emittance of the common mineral quartz to a perfect blackbody when both are at thermal equilibrium
at a given temperature (here, at 600 °K).
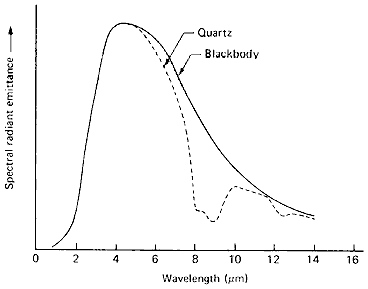
From T.M. Lillesand and R.W. Kieffer, Remote Sensing and Image Interpretation, 2nd Ed., © 1987. Reproduced by permission of J. Wiley & Sons, New York.
The sharp decrease in in the 8-10 µm region noted for quartz and other silicates is a "reststrahlen" effect (decreased emission)
related to thermally induced stretching vibrations associated
with silicon-oxygen bonds. In general, for opaque materials,
, where rho is the materials optical reflectance. Thus, as
, with high reflectance of radiation (poor absorptance),
the emittance will be low (thus, thermal radiation decreases).
Water, which has a high emissivity in the thermal infrared in
the 8-10 µm interval, is a poor reflector over that range; quartz (and many silicate
rocks) is a good emitter at lower thermal wavelengths but poor in this
interval. From this, one might predict that rock surfaces would
appear darker than water in the 8 -10 µm interval but this holds only
for certain conditions, as we will shortly see.
The radiant (sensed) temperature differs from a body's kinetic
(internal) temperature according to the relation ; for real bodies (known as gray bodies) radiant temperatures are always
less than kinetic temperatures. Thus, checking the figure below, the radiant temperature
is significantly higher for a blackened surface (high
) than for a shiny surface (lower
), even if the two materials are at the same kinetic temperature.
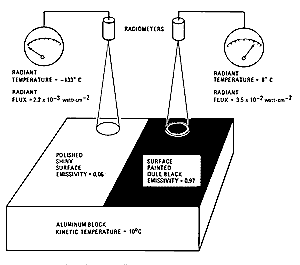
From F.F. Sabins, Jr., Remote Sensing: Principles and Interpretation. 2nd Ed., © 1987. Reproduced by permission of W.H. Freeman & Co., New York City.
Code 935, Goddard Space Flight Center, NASA
Written by: Nicholas M. Short, Sr. email: nmshort@epix.net
and
Jon Robinson email: Jon.W.Robinson.1@gsfc.nasa.gov
Webmaster: Bill Dickinson Jr. email: rstwebmaster@gsti.com
Web Production: Christiane Robinson, Terri Ho and Nannette Fekete
Updated: 1999.03.15.