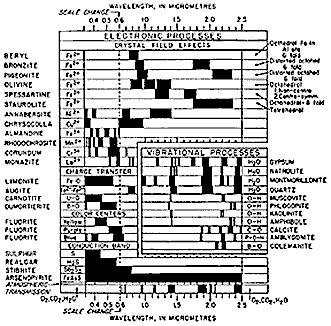
The causes of absorption are multifold and different ones may manifest in the same material. We shall now examine rather briefly each of the more common processes - which depend on electron behavior in an atom's electronic shells and on bond characteristics during interactions with photons. As a visual guideline to several of these processes, scan the chart below, and return to it as needed during the ensuing treatment. Note that the dark (black) bars represent principal absorption bands in various mineral species.

Electronic processes involve absorption of photons of specific energies (hence, wavelengths) that bring about a "jump" of electrons from a lower energy state to an electronic shell in which the energy state is higher. A photon may then be emitted if the electron returns to a lower state. For shared electrons, the energy jump spreads out over a range of values, producing "energy bands".
A common electronic movement results from Crystal Field disturbance. A crystal field describes the effects of perturbation of "d" orbitals of a transition metal (Fe, Cr, Ni, Co, Ti, V, etc.) distributed within the atomic lattice making up a crystal. This metal cation is subjected to the electric field imposed by surrounding anions (negatively charged) or dipolar groups (ligands). The charge symmetry is thus distorted. Five d orbitals, with different spatial configurations, are available for occupancy by electrons. These orbitals have different energy levels (i.e., are split). For a jump to occur, the amount of energy is quantized, that is, will require a photon of a particular energy equal to the difference between the specific levels of the higher and lower states. The electron involved may remain in the higher state (metastable), producing a characteristic absorption whose wavelength is fixed by the energy difference defining it. Crystal field theory is important in determining the color and magnetic properties of minerals and other substances containing transition elements.
Iron-bearing minerals, such as Pyrite (FeS2) and Magnetite (Fe3 O4), are telling examples of transition metal influence on their spectra. Many minerals contain both divalent iron (Fe2+) and trivalent iron (Fe3+). Thus, the valence state, the ionic coordination number, and site symmetry, the ligand geometry, and other factors determine the permissible energy increments.
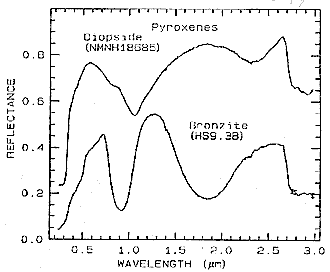
The influence of iron is evident in this next spectral plot through parts of the VNIR and SWIR ranges of two pyroxenes. Diopside (CaMgSi2O6) contains almost no Fe. Bronzite ([Mg,Fe]SiO3) has Fe but no Ca. The presence of Fe2+ causes two absorption bands, near 1 and 2 µm, to deepen and shift notably towards lower wavelengths.
A variant of this is called Charge Transfer Absorption. Here, photo absorption brings about an actual relocation of an ion (commonly, of the transition elements) to another position in the ionic or ligand crystal structure. The result is one to a series of deep absorption bands, many occurring in the ultraviolet wavelength region into the blue and green. The reds of Hematite (see plot on previous page) result from this type of absorption in which the spectrum now shows a strong reflectance peak around 0.75 µm set off by absorption on either side.
A third process utilizes Conduction Bands. Here, electrons move freely through the crystal lattice at a higher energy level (the conduction level) but tend to stay attached to their individual atoms at lower levels (Valence Bands). Photon excitation raises the level through this Band Gap. Not typical of metal ion behavior, this process is associated with semi-conductors.
Some minerals, including Fluorite (CaF2) show a range of characteristic colors controlled by absorption at Color Centers. These centers can develop from crystal structure defects, or commonly, from impurities. Such anomalies cause absorption at certain wavelengths leaving one or more reflectance peaks that combine to give the observed colors. Fluorite is normally purple to blue but green, yellow, and brown varieties are known. The phenomenon contributes to this mineral's ability to undergo electron jumps in response to ultraviolet radiation in which the return to the ground state gives off a color glow or fluorescence.
Separate from electronic processes are the Vibrational processes. These involve the bonds in a lattice or a molecular compound. At certain energies, the atomic units held by (usually covalent) bonds will be set into motion, either as a back and forth vibration and/or as a rotation. The frequencies at which absorption ensues are governed by the strengths of the bonds and the masses of atoms or ions participating in the movement. (Solids experience smaller vibrations than liquids or gases.) Given a molecule composed of N atoms, there are 3N - 6 normal modes of vibration. Each of these constitutes a fundamental vibration. There can be additional vibrations at lower frequencies (fixed multiples of the fundamentals) which comprise overtones; these normally are weaker.
The majority of vibrational absorptions occur in non-metallic materials and show up throughout the infrared (both short and mid-wavelength ranges). They tend to produce rather diverse and complex spectra. Some troughs are fundamentals; others are overtones. In some instances, two or more modes of vibration are possible at the same frequency(ies).
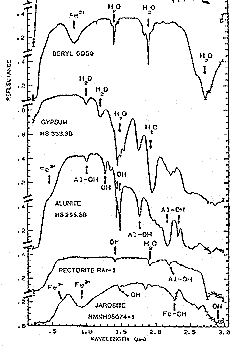
The figure below shows mainly vibrational absorptions for several silicate and sulphate non-metallic minerals. Some of the absorption features are due to H2O and others to hydroxyl (OH) bonded to Fe or Al. Not shown are Calcite and Dolomite spectra that show multiple absorption troughs in the 2.0 to 3.0 µm interval.
Code 935, Goddard Space Flight Center, NASA
Written by: Nicholas M. Short, Sr. email: nmshort@epix.net
and
Jon Robinson email: Jon.W.Robinson.1@gsfc.nasa.gov
Webmaster: Bill Dickinson Jr. email: rstwebmaster@gsti.com
Web Production: Christiane Robinson, Terri Ho and Nannette Fekete
Updated: 1999.03.15.