The ElectroMagnetic Spectrum
Most texts on remote sensing begin by giving the reader a survey
of the main principles needed to build a theoretical background
(mainly in the physics of radiation). While it is important to
have such a framework if one wishes to pursue many aspects of
remote sensing, we will not delve into this profound subject in
much detail at this point (the user interested in more depth of
understanding is referred to the three chapters from The Landsat Tutorial Workbook ). Instead, we will offer here only an outline survey of the basics
of relevant electromagnetic concepts.
The fundamental unit involved in electromagnetic phenomena is the photon (one form of quanta, as considered in quantum physics). This is the subatomic particle which comprises radiation given off by matter when it is excited thermally, or by nuclear processes (fusion, fission), or by other radiation. Photons, which are without mass, in transfer move at the speed of light: 300,000 km/sec (186,000 miles/sec). As such, these particles also move as waves - hence, they have a "dual" nature. These waves follow a pattern described in terms of a sine (trigonometric) function, as shown in two dimensions in the figure below.
The distance between equivalent points on a wave train is its wavelength. The total number of equivalent points that pass by a reference in a second is expressed by the frequency of that wave (associated terms include cycles per second; hertz). A given photon has a certain amount of energy. This packet of energy (in ergs) is determined by the general Planck equation:
![]()
where his the Planck constant (6.6260... x 10-34 Joules-sec) *and vis a Greek letter representing frequency. Photons travelling at
higher frequencies are therefore more energetic. If a material
under excitation experiences a change in energy level from a higher
level E2 to a lower state E1, the above formula would be restated as:
![]()
where v has some discrete value determined by (v2 - v1); in other words, a particular energy change is characterized
by production of emitted radiation (photons) that has some specific
frequency v and a corresponding wavelength 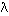 .
.
Wavelength is the inverse of frequency (higher frequencies associate with shorter wavelengths; lower with longer), as given by the relationship:
![]()
where cis the constant that expresses the speed of light, so that the Planck equation can also be written as
![]()
Code 935, Goddard Space Flight Center, NASA
Written by: Nicholas M. Short, Sr. email: nmshort@epix.net
and
Jon Robinson email: Jon.W.Robinson.1@gsfc.nasa.gov
Webmaster: Bill Dickinson Jr. email: rstwebmaster@gsti.com
Web Production: Christiane Robinson, Terri Ho and Nannette Fekete
Updated: 1999.03.15.